
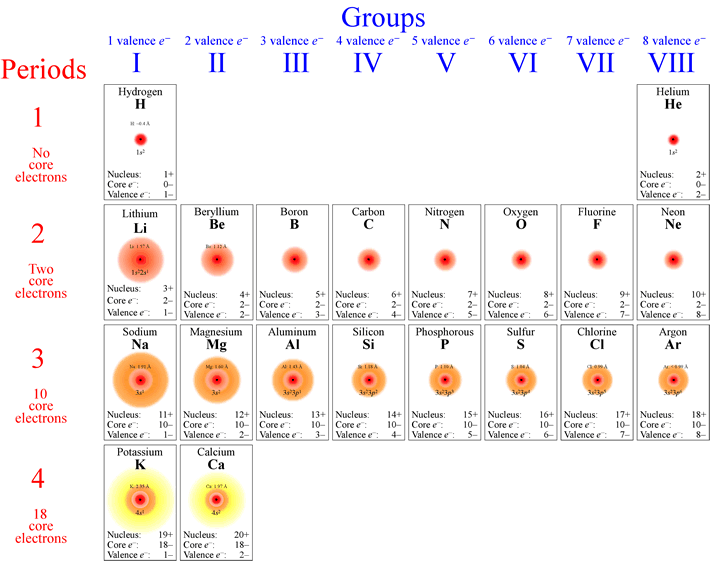
When the atomic size or atomic radius is large, the E.A will be smaller. The relationship between E.A and atomic size is given below: There are the following factors that affect electron affinity: Atomic size Exceptions to the trends of Electron Affinity.Here, the value of electron affinity decreases, and the addition of electrons becomes more and more difficult. Hence, it is an endothermic process.įor example, E.A for O -1 is -142 KJ., and for O 2- is +844 KJ. The negative ion repels the incoming electron. However, when a second electron is added to a uni-negative ion. So the first electron affinity values are always negative.

Electron affinity (E.A) is the amount of energy released when an electron is added to a neutral gaseous atom or molecule to form a negative ion.


 0 kommentar(er)
0 kommentar(er)
